According to a recall notice issued by the US Food and Drug Administration, a blood pressure medication is being recalled because it may contain high levels of a cancer-causing impurity (FDA).
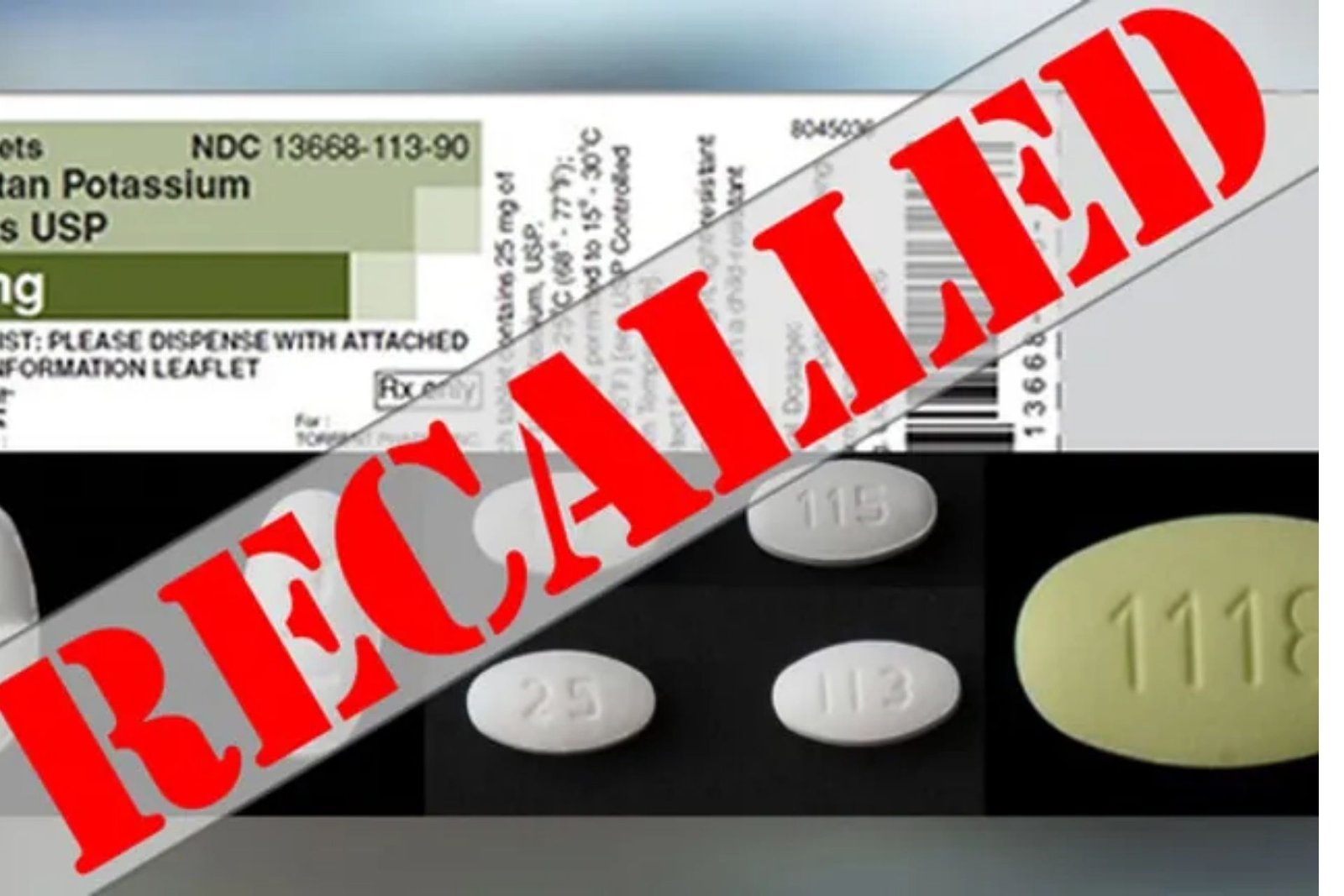
Lupin Pharmaceuticals Inc. is voluntarily recalling its Irbesartan and Hydrochlorothiazide tablets from pharmacies.Based on laboratory test results, certain tested API batches exceeded the specification limit for the impurity, N-nitrosoirbesartan — a probable human carcinogen (a substance that may cause cancer).
Lupin is recalling all batches of Irbesartan Tablets USP 75mg, 150mg, and 300mg, as well as Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg, in the United States. Irbesartan Tablets USP, 75mg, 150mg, and 300mg, are packaged in 30- and 90-count bottles and distributed to wholesalers, drug chains, mail-order pharmacies, and supermarkets across the United States.

Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg, are packaged in 30- and 90-count bottles and distributed to wholesalers, drug chains, mail order pharmacies, and supermarkets across the United States.According to the recall notice, Lupin Pharmaceuticals Inc. is notifying its wholesalers, distributors, drug chains, mail order pharmacies, and supermarkets by phone and through recall notification, and is arranging for the return of all recalled product lots.
Patients who are taking the medications are advised to continue taking them and to contact their pharmacist, physician, or medical provider for advice on alternative treatment options. Inmar Rx Solutions Inc. can be reached at 855-769-3988 or 855-769-3989 if consumers, wholesalers, distributors, or retailers have any questions about this recall. Return the recalled lots to Inmar Rx Solutions, Inc. for reimbursement; the lot number can be found on the side of the bottle label.
______
Cancer | Don’t forget to follow us on Twitter @njtimesofficial. To get the latest updates