Denmark has stopped giving the Oxford–AstraZeneca Covid vaccine due to concerns about rare blood clot events, the first European country to do so entirely.
The switch would delay the country’s vaccination schedule by several weeks.
Last week’s European Medicines Agency drug watchdog reported a potential association with clots, but said Covid-19’s risk of dying was much greater.
Several European countries had briefly postponed the jab.

Most have resumed AstraZeneca vaccines, but also with limits to older age groups.
For similar reasons over clotting, the US, Canada, and the EU suspended the Johnson & Johnson vaccine on Tuesday.
South Africa has also paused its use, despite Johnson & Johnson being its favoured vaccine due to its efficacy against the South African version.
Blood clot side effects are very rare for AstraZeneca and Johnson & Johnson.
Blood clots are an ‘AstraZeneca side effect’
What to do about vaccine safety
The World Health Organization (WHO) has criticized the EU vaccine roll-out for being too sluggish, and there are fears that this latest setback might throw it into more chaos.
Both vaccines function similarly, known as adenoviral vectors.
Why stop Denmark’s AstraZeneca vaccine?
Danish officials said all 2,4 million AstraZeneca vaccine doses will be withheld before further notice.
The Danish Health Authority said studies showed higher-than-expected blood clot incidence after doses, affecting around one in 40,000 people.
It comes after two cases of Denmark thrombosis linked to vaccinations, AFP published. One case, a 60-year-old woman, was fatal.
Director-General Soren Brostrom said it was a “difficult decision,” but Denmark had other vaccines available and the epidemic was currently under control.
“Covid-19’s upcoming target groups for vaccination are less likely to become seriously ill,” he said. “We must balance this against the fact that we now have a proven risk of serious adverse effects from AstraZeneca vaccination, even if the absolute risk is slight.”
However, at another time, the authorities said they couldn’t rule out using it.
During the press conference, Denmark’s Medicines Agency’s chief, Tanja Erichsen, fainted and was taken to hospital as a precaution. The department later tweeted she’d recovered.
Nearly one million people were vaccinated in Denmark, with around 150,000 getting the AstraZeneca jab. Pfizer/BioNTech and Moderna are also used.
How does adenovirus work?
Adenoviruses in humans and other animals. Scientists use a modified version of one of these adenoviruses, known as a vector, to give cells essential instructions, according to the US Centers for Disease Control (CDC).
They function by entering cells and using machinery from the cell to create a harmless piece of virus that triggers Covid-19, known as spike protein. The cell then recognises that there is no spike protein, which activates the immune system to fight back against what it feels is infection.
This process helps our bodies to learn how to protect us from Covid-19, the CDC says.
Regulators are now studying whether an irregular immune response to adenovirus vaccines causes uncommon but serious blood clotting incidences.
An official from the U.S. Food and Drug Administration told Reuters that the cases of blood clots linked to the Johnson & Johnson vaccine were “very close” to those linked to the AstraZeneca vaccine.
The US suspended the Johnson & Johnson vaccine rollout after six women under 50 formed unusual blood clots after the injection. In the UK, 30 people developed rare blood clots and seven of them died after receiving the Oxford-AstraZeneca vaccine, out of 18 million vaccinated.
What do other countries do?
Some European countries have restricted the use of adenovirus vaccines to older people less affected by the unusual condition of blood clotting.
After the Danish announcement, France said it considered the AstraZeneca vaccine an “essential weapon.”
“This vaccine must continue to be deployed. It’s a good vaccine and works, “the French spokesperson said.
France also wants to offer the Johnson & Johnson vaccine to everyone over 55, the spokesman said. The nation has issued 200,000 doses. Belgium will also offer its doses, while Greece and Italy will not.
Is Europe’s decision-making Oxford jab flawed?
Is Oxford AstraZeneca effective vaccine?
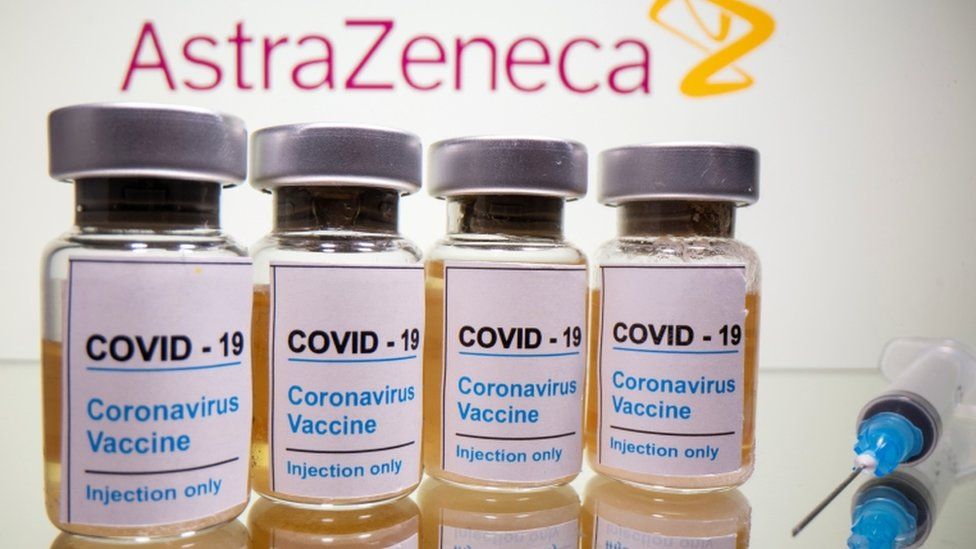
Meanwhile, Czech Deputy Prime Minister Jan Hamacek said he had ordered Denmark’s Czech ambassador to try to purchase the 2.4 million AstraZeneca vaccine doses the Danes would no longer need.
Mr. Hamacek said he would also fly to Moscow to arrange deliveries of the Russian Sputnik V vaccine, another adenovirus vaccine, once EMA approves its use.
The Sputnik V vaccine maker, the Gamaleya Center, told the BBC it had found no cases of blood clots related to its jab. Both adenovirus-based vaccines were “different and not directly comparable.”
Denmark was the first country to postpone AstraZeneca in March. Numerous other European countries followed.
More Pfizer-BioNTech doses
In a separate development, the European Commission said in the coming weeks, Pfizer-BioNTech will supply an additional 50 million doses to the EU.
Pfizer-BioNTech is an RNA vaccine, a modern form of vaccine that uses genetic material to teach cells how to copy the virus that causes Covid-19, activating an immune response.
Commission President Ursula von de Leyen also said that the EU was negotiating a new contract with Pfizer-BioNTech to supply 1.8 billion doses in 2022 and 2023, all manufactured within the EU.
In the EU, 27 million citizens have been completely vaccinated.
AstraZeneca | Don’t forget to follow us on Twitter @njtimesofficial. To get latest update