Exploration Square declared on July 19, 2021, it had removed a non-peer-evaluated preprint concentrate on July 14, 2021, ‘because of a declaration of concern conveyed straightforwardly to our staff.’
The examination, Efficacy, and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic, concerns were first raised by the British clinical understudy Jack Lawrence, as per The Guardian.
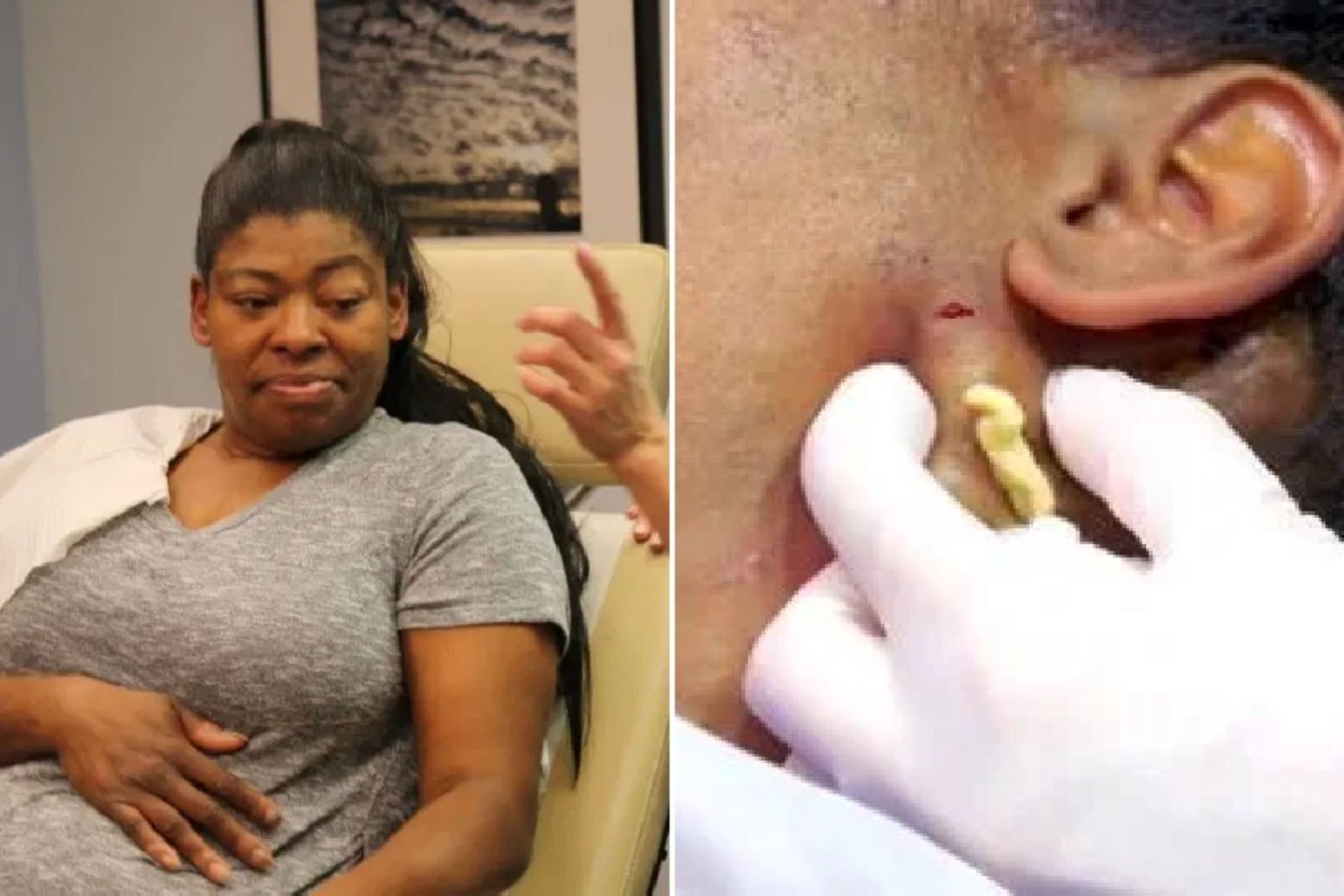
Ivermectin is anything but an enemy of viral medication for treating infections. What’s more, ivermectin arrangements for creatures are totally different from those endorsed for people.
The U.S. FDA has gotten reports of COVID-19 patients who have needed clinical help and been hospitalized after self-sedating with Ivermectin.
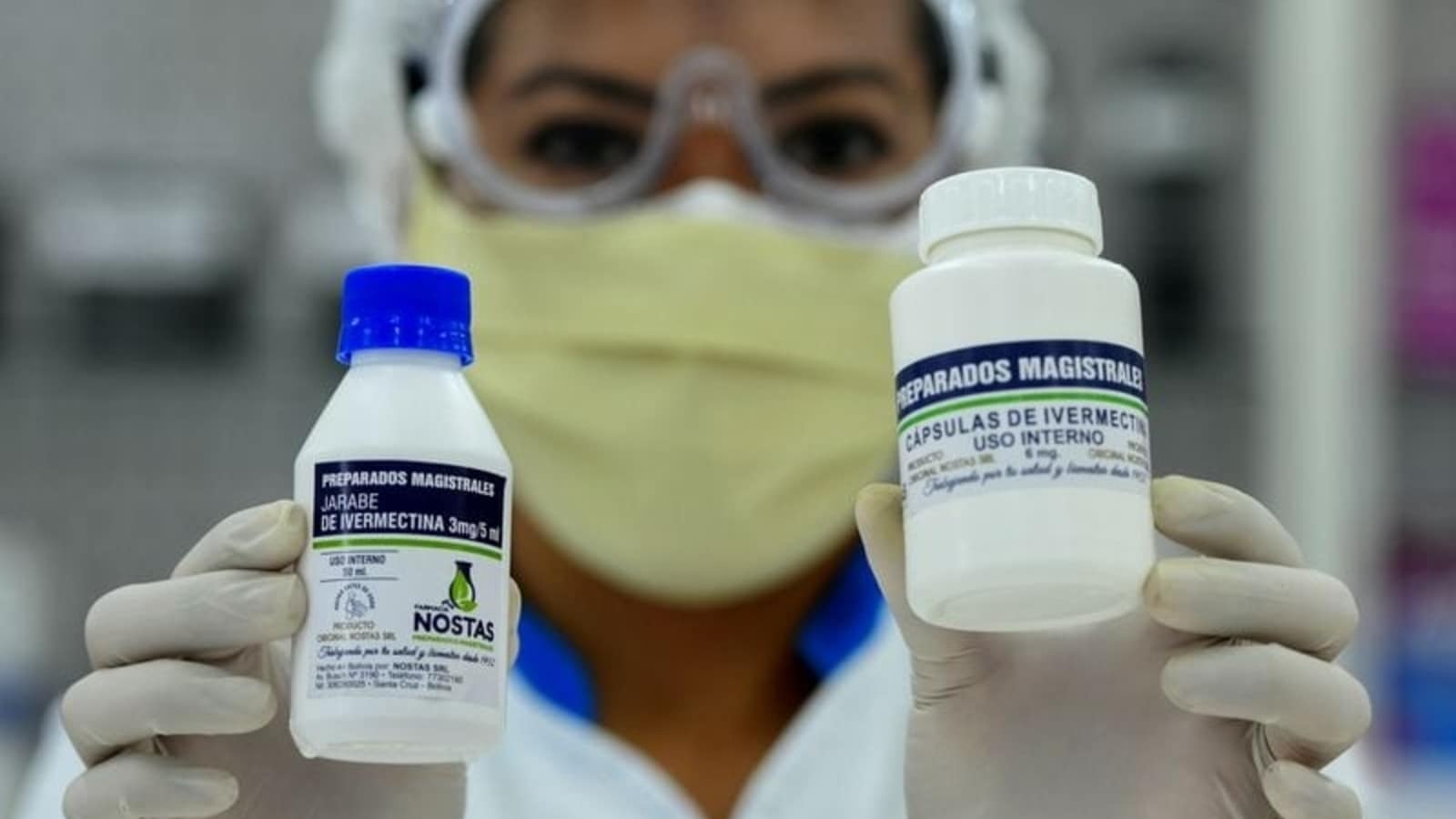
The FDA endorses ivermectin tablets to treat individuals with intestinal strongyloidiasis and onchocerciasis, two conditions brought about by parasitic worms.
Also, there are effective plans for head lice and skin conditions like rosacea.
In the event that you have a remedy for ivermectin for a FDA-supported use, take it precisely as endorsed, says the FDA.
Never use drugs planned for creatures on yourself.
A preprint study is a primer rendition of a composition that has not finished the companion survey measure at a diary. Exploration Square doesn’t direct companion survey prior to posting preprints.
The preprint posting ought not be deciphered as a support of its legitimacy or reasonableness for spread as set up data or for directing clinical practice.
___________________
Ivermectin | Don’t forget to follow us on Twitter @njtimesofficial. To get the latest updates