Doctors may have discovered why the COVID-19 vaccine of AstraZeneca can in very rare cases cause life threatening coagulation.
The discovery in a couple of online reports published on Friday in the German New England Journal may be a key factor in the global development of the AstraZeneca vaccine, helping to develop effective side effects therapies and providing insights into how the vaccine is improved and solved, say experts.
However, this may also hinder efforts to have the vaccine approved in the USA, where three vaccines already exist.
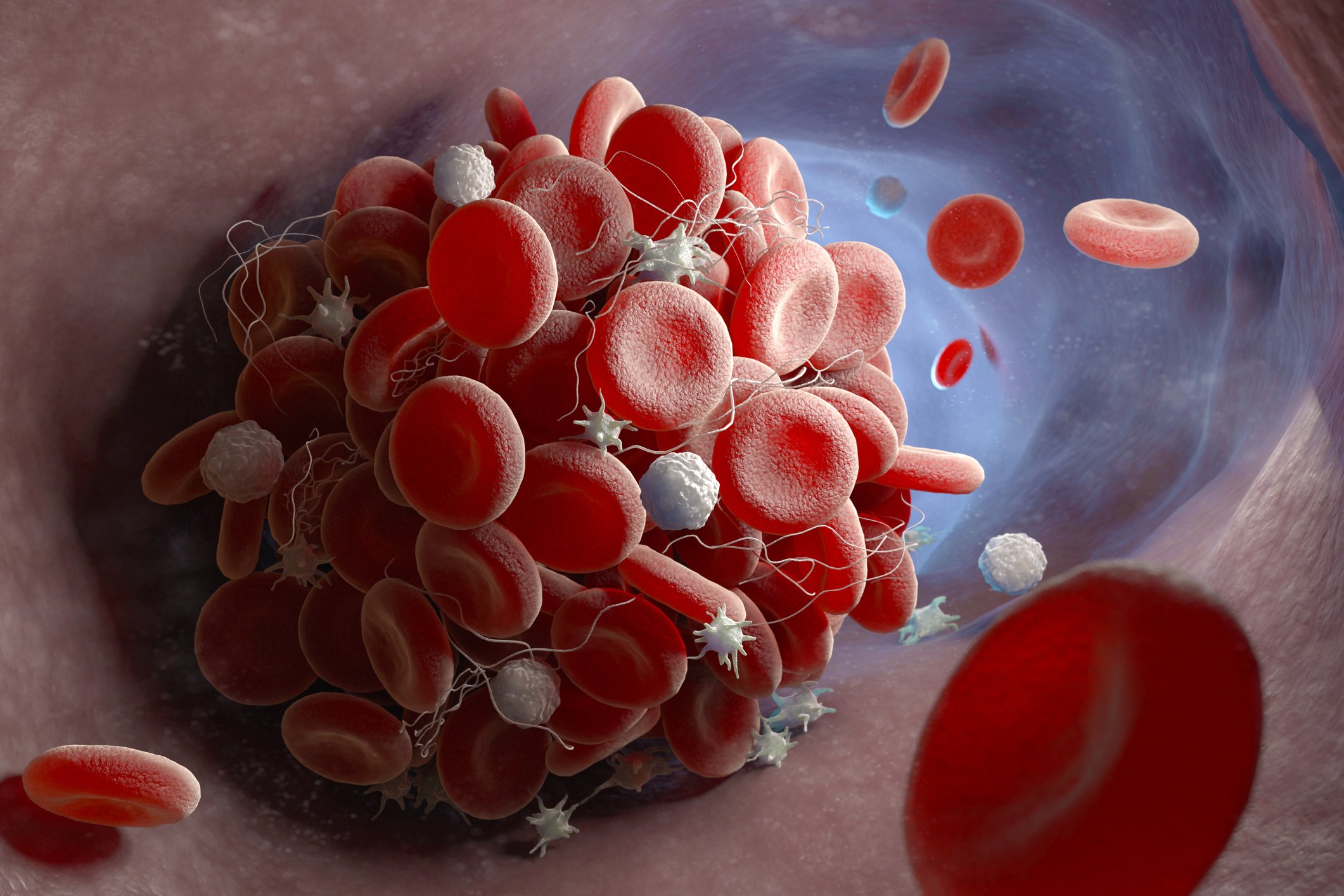
The vaccine for AstraZeneca appears to cause certain people to develop antibodies that target a human body protein called a platelet factor 4 (PF4), which stimulates platelets into action and triggers a co-author, explains Dr. Theodore Warkentin, a Pathology and Molecular Medicine Professor at McMaster University in Ontario.
“This is a vaccine-led antibody, and this leads to some unusual blood clotting in some circumstances,” Warkentin said.
The phenomenon is similar to a rare medicinal side-effect caused by heparin thinner, called thrombocytopenia caused by heparin, said Warkentin.
The clotting adverse effects of the vaccine are so rare that it was decided by the European Medicines Agency (EMA) and the UK Regulatory Agency for Medicines and Healthcare Products that the benefits of it prevail to the greater extent than the risk, AstraZeneca noted in a statement.
However, the label of the vaccine will be updated as an extremely rare potential side effect in blood clotting.
“AstraZeneca has cooperated actively with the regulators to make such changes to the information on the product and is already understanding the individual cases, epidemiology and possible mechanisms which could explain the extremely rare events,” the company declaration said.
EMA reported 169 cerebral clotting cases and 53 abdominal clotting cases of some 34 million AstraZeneca doses managed across Europe, according to Reuters, as of April 4.
In the UK, 19 people have been killed by severe vaccine-related blood clots, CNN reported.
Clotting cases mirror those sometimes triggered by blood thinner heparin

One of the new AstraZeneca vaccine reports focuses on 11 patients who developed severe clotting after receiving the vaccine in Germany and Austria, while the other reviewed the cases of five health workers aged 32-54 who developed the adverse events.
Tests showed that, even though none were given blood thinners, all patients developed PF4 blood clotting complexes similar to heparin.
__________________________________________________________________________________________________________
VACCINE | Don’t forget to follow us on Twitter @njtimesofficial. To get latest update