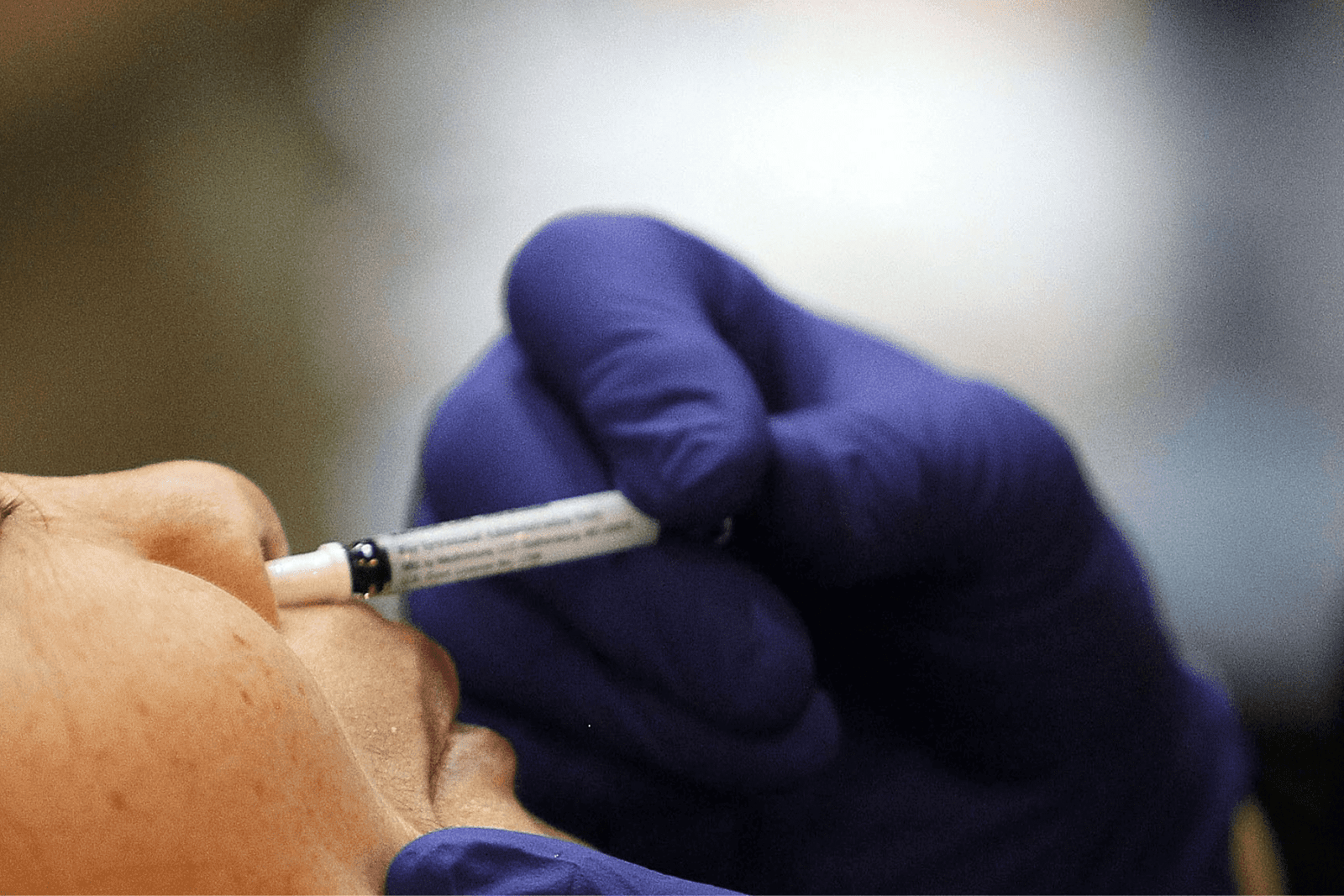
According to hospital officials, a groundbreaking clinical trial of a nasal vaccine for Alzheimer’s disease that has been in the works for decades is set to begin at Brigham and Women’s Hospital in Boston.
The trial’s goal is to assess the safety and efficacy of a nasally delivered vaccine designed to prevent and slow the progression of Alzheimer’s disease (AD).Dr. Howard L. Weiner, co-director of Brigham’s Ann Romney Center for Neurological Diseases, called the start of the first human trial of an Alzheimer’s disease nasal vaccine a “remarkable milestone.”
The clinical trial will include 16 participants between the ages of 60 and 85 who have early symptomatic Alzheimer’s but are otherwise healthy, according to a press release from the hospital.Each person will be given two doses of the vaccine, one week apart. According to the release, the participants will enrol through the Ann Romney Center.
According to the researchers, the vaccine contains a substance called Protollin, which stimulates the immune system.
The substance has also been found to be safe in other vaccines.”According to the release, “protollin is designed to activate white blood cells found in lymph nodes on the sides and back of the neck to migrate to the brain and trigger clearance of beta-amyloid plaques — one of the hallmarks of Alzheimer’s disease.”
The primary goal of the phase I trial, according to the researchers, will be to “determine the safety and tolerability of the nasal vaccine,” as well as to observe how Protollin affects participants’ immune responses, including white blood cells.The nasal vaccine, according to Weiner, is a “unique approach” because it affects a person’s immune system.
He spoke enthusiastically about the vaccine trial launch with CBS News.”Potentially, it could be a treatment for people who have the disease, but more importantly, it could be something to prevent people from getting the disease,” Weiner said.Tanuja Chitnis, MD, professor of neurology at Brigham and Women’s Hospital and the trial’s principal investigator, stated: “For the past two decades, there has been mounting evidence that the immune system plays a critical role in the elimination of beta-amyloid.
“This vaccine uses a novel arm of the immune system to treat Alzheimer’s disease,” she explained.”Research in this area has paved the way for us to pursue a whole new avenue for potentially treating not only Alzheimer’s disease, but also other neurodegenerative diseases,” Chitnis added.
Protollin is developed, manufactured, and commercialised by I-Mab Biopharma and Jiangsu Nhwa Pharmaceutical.
_______
Alzheimers | Don’t forget to follow us on Twitter @njtimesofficial. To get latest updates